PRESENTATION MATERIALS FOR KEY OPINION LEADER CALL ON DECEMBER 17, 2020
Published on December 17, 2020
Exhibit 99.2

1 Scientists Enabling Healthcare COVID - 19: The Vaccine is Out, Now What Does that Mean for Testing ? KOL Event December 17, 2020

2 Forward Looking Statements Except for historical information, the matters discussed in this presentation may be considered "forward - looking" statements within the meaning of Section 27 A of the Securities Act of 1933 , as amended, and Section 21 E of the Securities Exchange Act of 1934 , as amended . Such statements include declarations regarding the intent, belief or current expectations of the Company and its management, including those related to cash flow, gross margins, revenue, and expenses which are dependent on a number of factors outside of the control of the Company including, inter alia, the markets for the Company’s products and services, costs of goods and services, other expenses, government regulations, litigation, and general business conditions . See Risk Factors in the Company’s Form 10 - K for the fiscal year ended July 31 , 2020 and Form 10 - Q for the period ended October 31 , 2020 . Investors are cautioned that any such forward - looking statements are not guarantees of future performance and involve a number of risks and uncertainties that could materially affect actual results . The Company disclaims any obligations to update any forward - looking statement as a result of developments occurring after the date of this presentation .

3 3 Elazar Rabbani, PhD Dr . Elazar Rabbani, PhD is an Enzo Biochem founder and has served as the Company's Chairman of the Board and Chief Executive Officer since its inception in 1976 . Dr . Rabbani has authored numerous scientific publications in the field of molecular biology, in particular, nucleic acid labeling and detection . He is also the lead inventor of many of the Company's pioneering patents covering a wide range of technologies and products . Dr . Rabbani received his Bachelor of Arts degree from New York University in Chemistry and his Ph . D . in Biochemistry from Columbia University . He is a member of the American Society for Microbiology . He has published over 19 publications and has 110 U . S . Patents (with 125 foreign counterparts) .

Scientists Enabling Healthcare Introduction

5 5 +40% YoY Revenue Growth FY21E ~ $115M 445 employees operating on a global basis 450+ Patents and Patent Applications Global HQ in NYC with a worldwide distribution network Diagnostic Product Development and manufacturing under GMP compliance, CAP Accredited & CLIA Certified Company Snapshot “ Our assets, infrastructure and capabilities have most recently been directed to address a growing market need in the area of diagnostic products and services.”
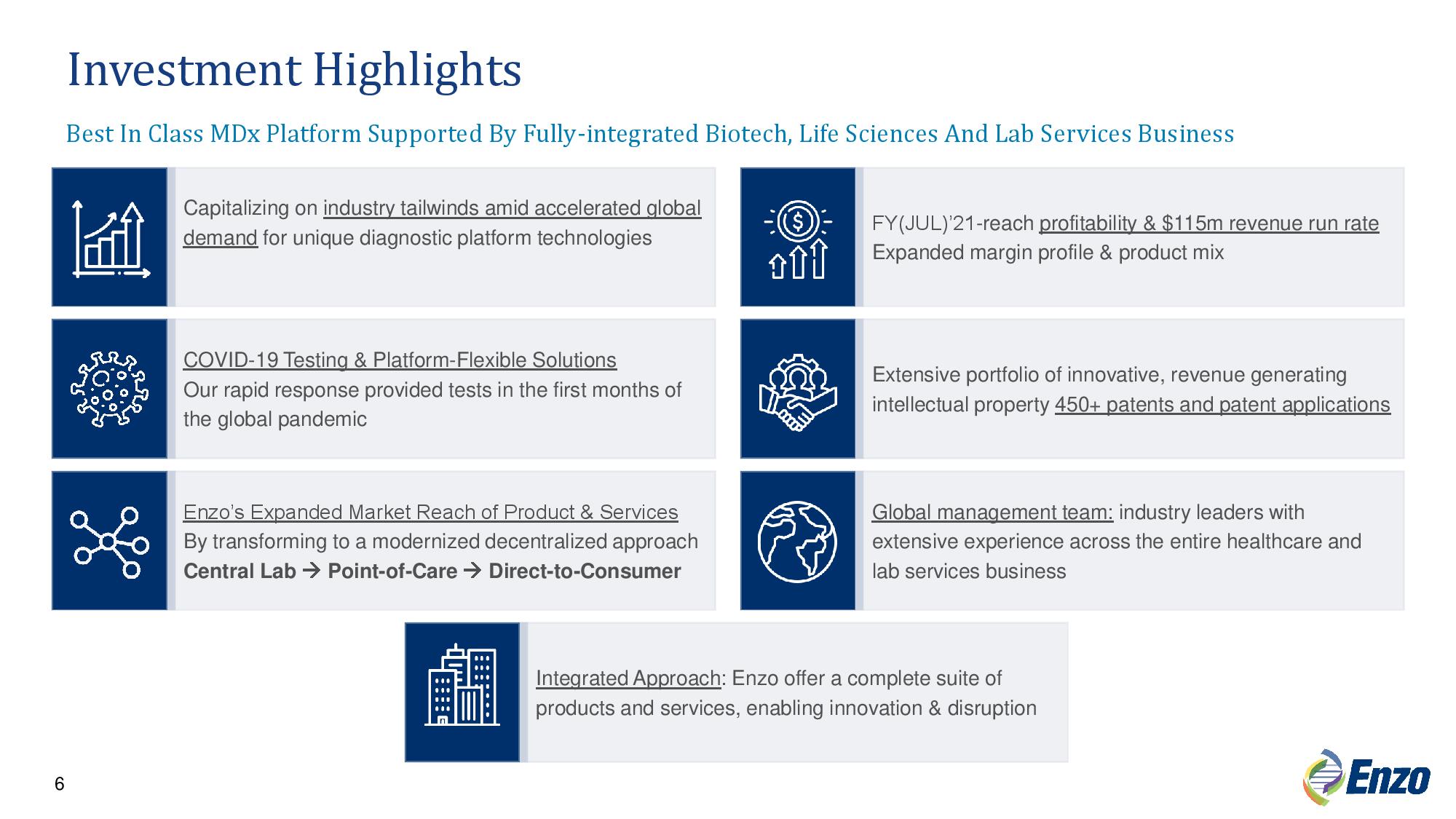
6 Capitalizing on industry tailwinds amid accelerated global demand for unique diagnostic platform technologies FY(JUL)’21 - reach profitability & $115m revenue run rate Expanded margin profile & product mix COVID - 19 Testing & Platform - Flexible Solutions Our rapid response provided tests in the first months of the global pandemic Extensive portfolio of innovative, revenue generating intellectual property 450+ patents and patent applications Integrated Approach : Enzo offer a complete suite of products and services, enabling innovation & disruption Global management team: industry leaders with extensive experience across the entire healthcare and lab services business Enzo’s Expanded Market Reach of Product & Services By transforming to a modernized decentralized approach Central Lab Point - of - Care Direct - to - Consumer Investment Highlights Best In Class MDx Platform Supported By Fully - integrated Biotech, Life Sciences And Lab Services Business

7 Extensive Capabilities Products & Services DEEPSEE ® FISH Probes AMPIPROBE ® Assays Molecular Diagnostics CYTAG ® CGH Labeling Genetics & Genomics Immunoassays POLY - VIEW ® PLUS Detection Anatomical Pathology

8 8 Bruce Hanna, PhD Bruce Hanna, PhD, has served as a Clinical Professor of Pathology and Clinical Professor of Microbiology at the New York University School of Medicine since 1979 and Adjunct Professor of Science at New York University College of Dentistry since 2010 . From 2006 to 2015 , he served on the ASM International Committee and WHO Global Committee ; from 2000 to 2012 , he served as the Editor of the Clinical Microbiology Review ; from 1982 to 2010 , he was a director of Clinical Microbiology and Immunology ; and from 2008 to 2010 he was Interim director of Pathology, Bellevue Hospital Center . Dr . Hanna earned a Bachelor of Science in Biology from Saint Bonaventure University, a Masters in Science in Microbiology from Northeastern University and a Ph . D . in Microbiology from Saint John’s University . Dr . Hanna’s post - doctorate work in Clinical Microbiology was at Mt . Sinai Hospital .
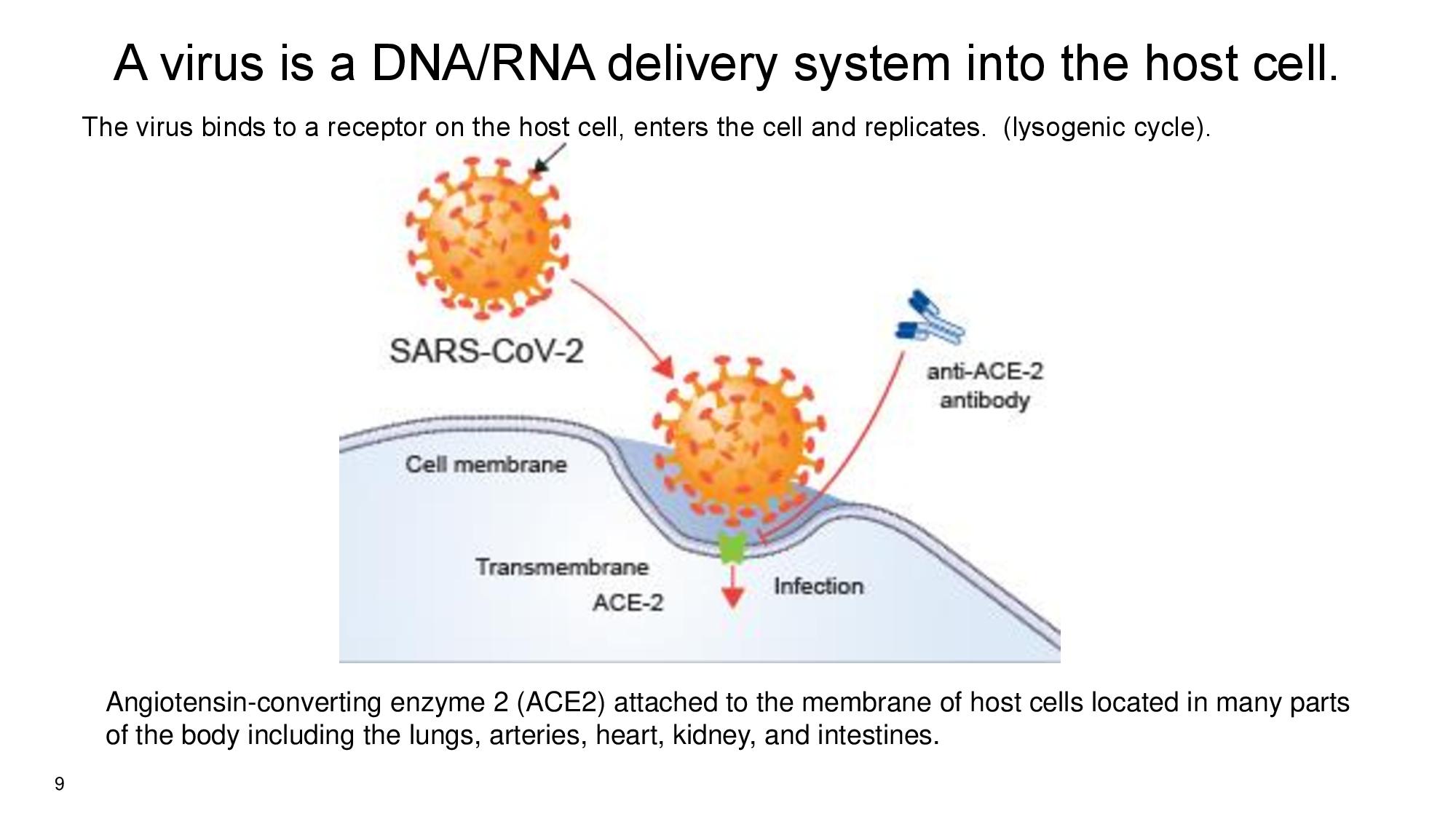
9 A virus is a DNA/RNA delivery system into the host cell. The virus binds to a receptor on the host cell, enters the cell and replicates. (lysogenic cycle). Angiotensin - converting enzyme 2 (ACE2) a ttached to the membrane of host cells located in many parts of the body including the lungs, arteries, heart, kidney, and intestines.
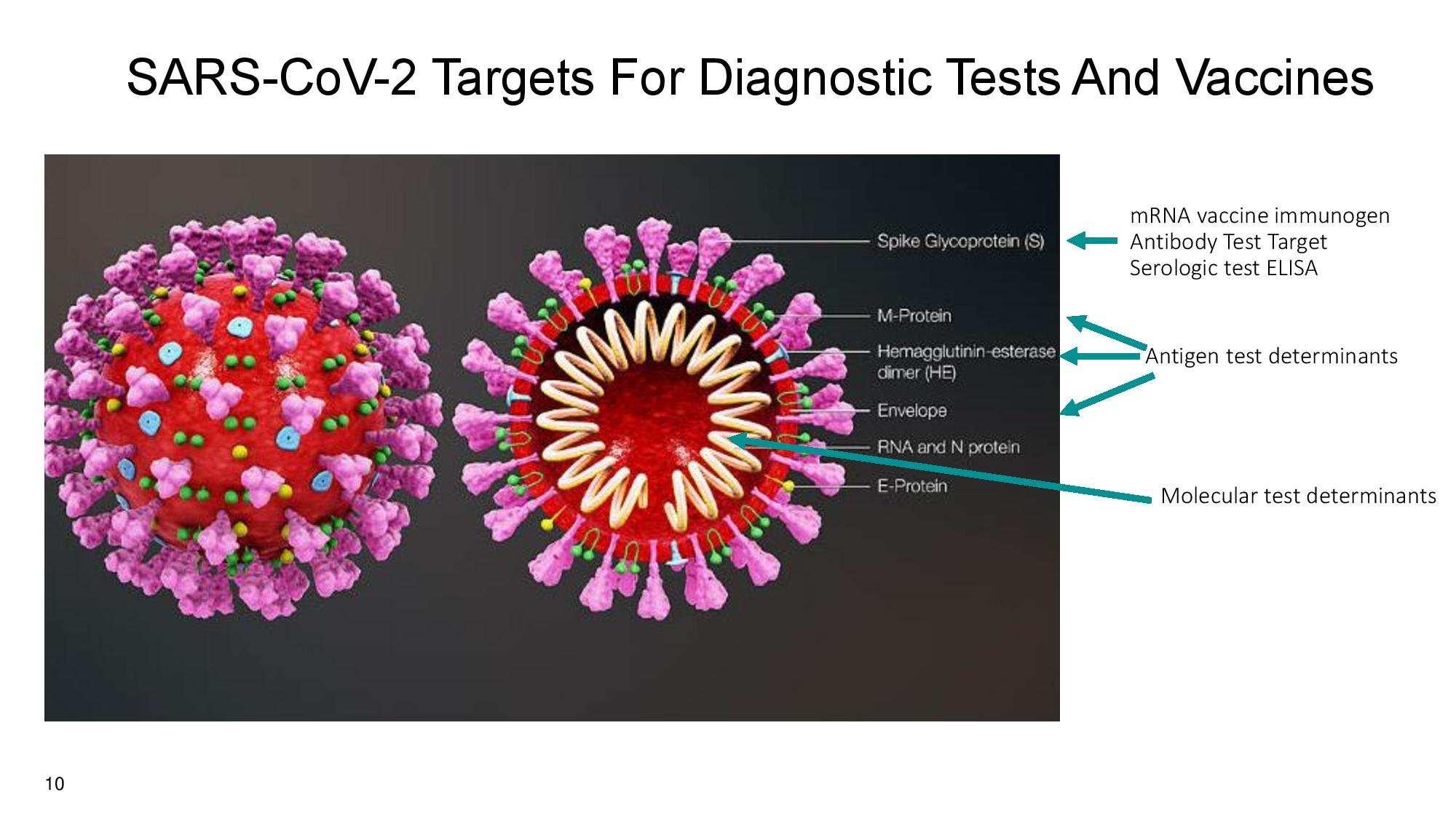
10 mRNA vaccine immunogen Antibody Test Target Serologic test ELISA Antigen test determinants Molecular test determinants SARS - CoV - 2 Targets For Diagnostic Tests And Vaccines
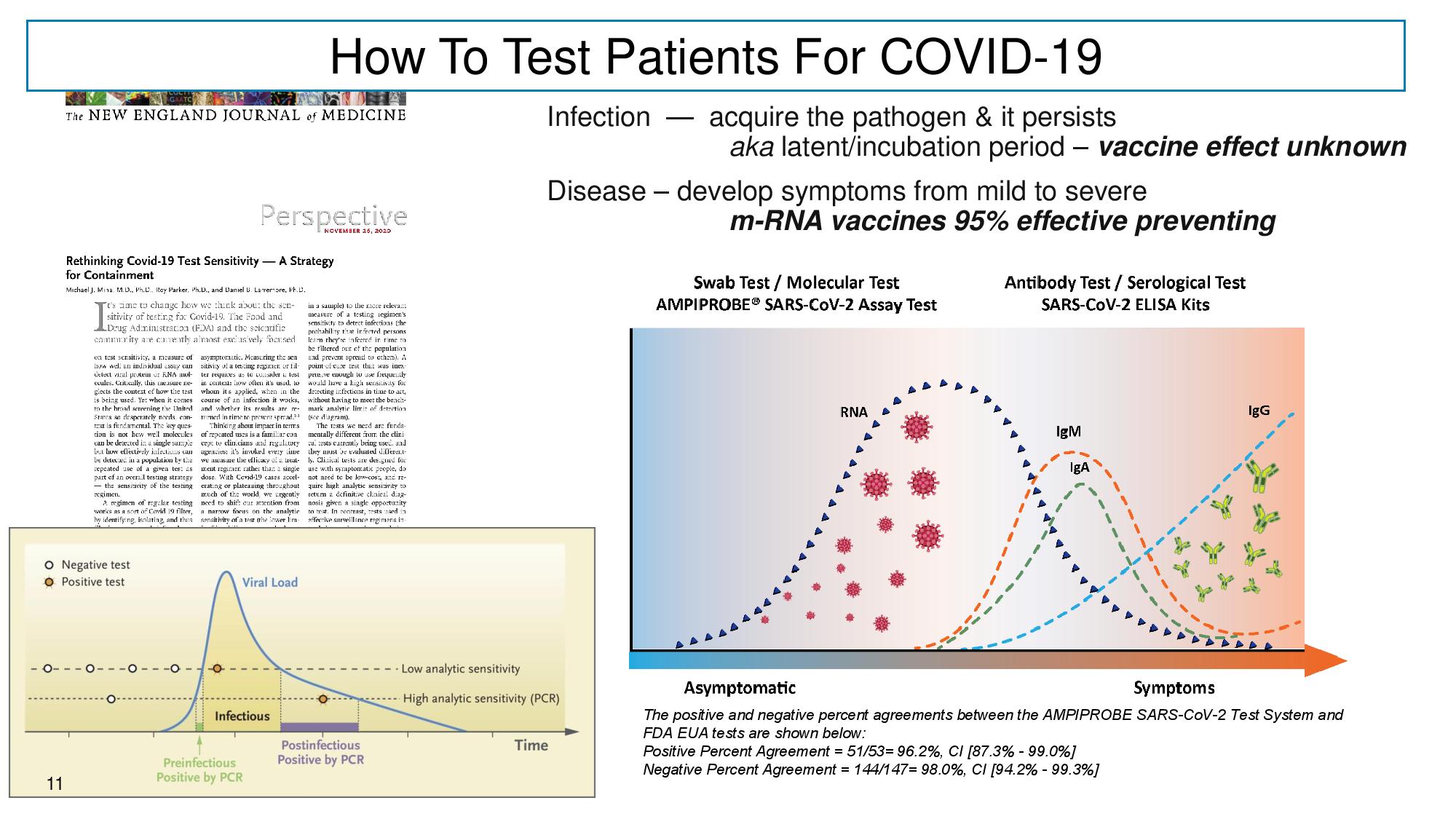
11 How To Test Patients Fo r COVID - 19 Infection — acquire the pathogen & it persists aka latent/incubation period – vaccine effect unknown Disease – develop symptoms from mild to severe m - RNA vaccines 95% effective preventing The positive and negative percent agreements between the AMPIPROBE SARS - CoV - 2 Test System and FDA EUA tests are shown below: Positive Percent Agreement = 51/53= 96.2%, CI [87.3% - 99.0%] Negative Percent Agreement = 144/147= 98.0%, CI [94.2% - 99.3%] 11
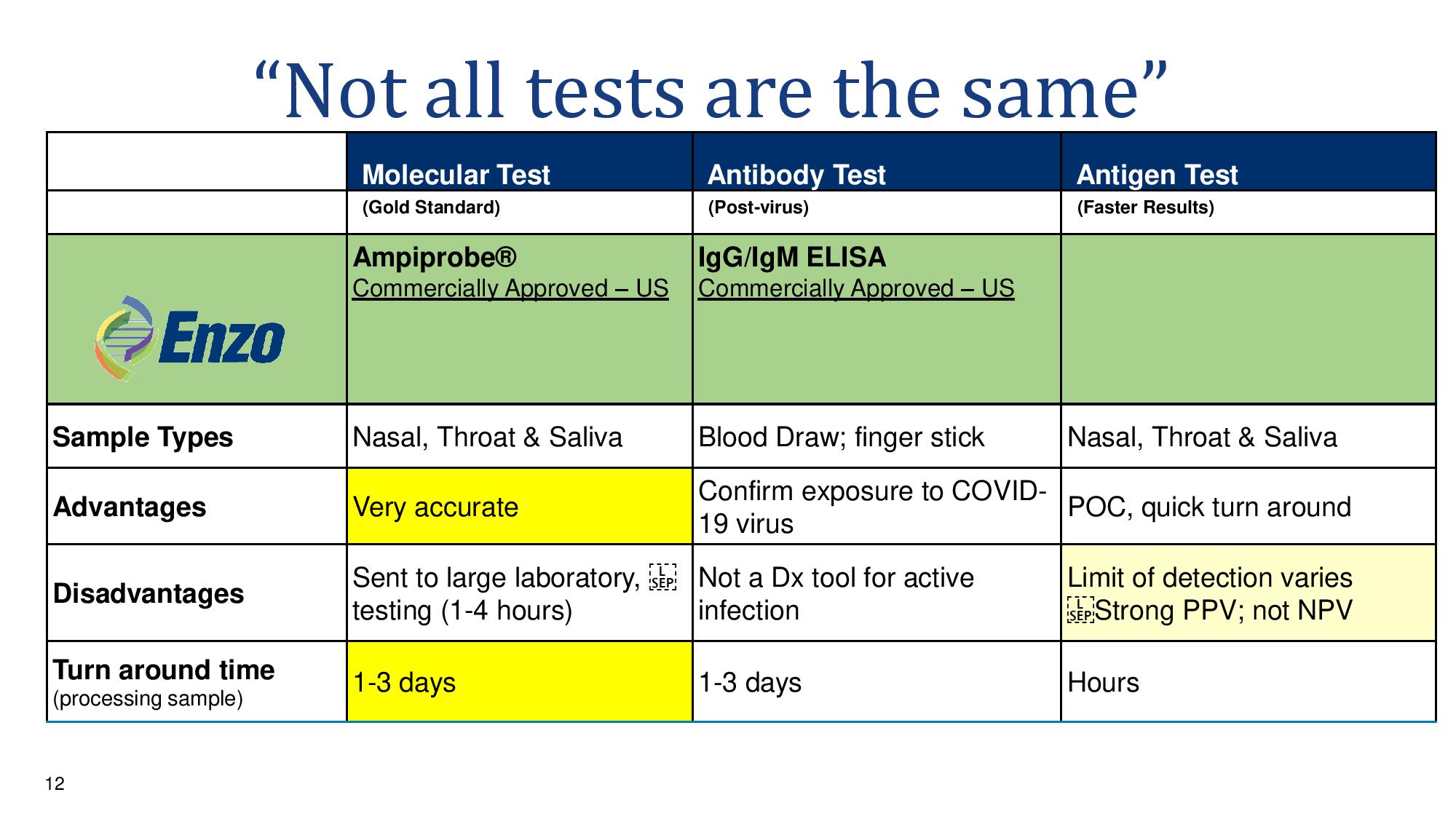
12 “Not all tests are the same” Molecular Test Antibody Test Antigen Test (Gold Standard) (Post - virus ) (Faster Results) Ampiprobe ® Commercially Approved – US IgG/IgM ELISA Commercially Approved – US Sample Types Nasal, Throat & Saliva Blood Draw; finger stick Nasal, Throat & Saliva Advantages Very accurate Confirm exposure to COVID - 19 virus POC, quick turn around Disadvantages Sent to large laboratory, testing (1 - 4 hours) Not a Dx tool for active infection Limit of detection varies Strong PPV; not NPV Turn around time (processing sample) 1 - 3 days 1 - 3 days Hours
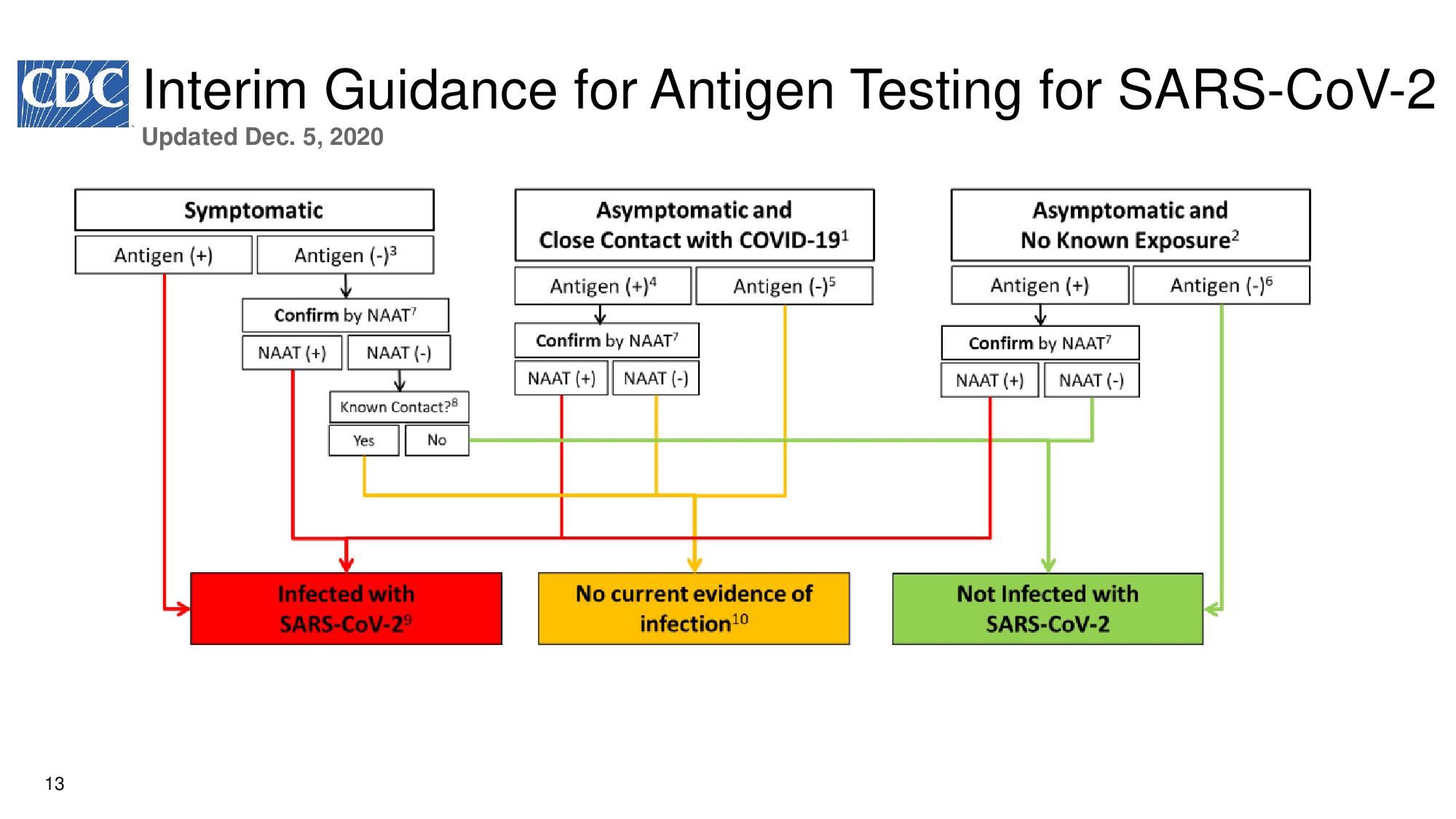
13 Interim Guidance for Antigen Testing for SARS - CoV - 2 Updated Dec. 5, 2020
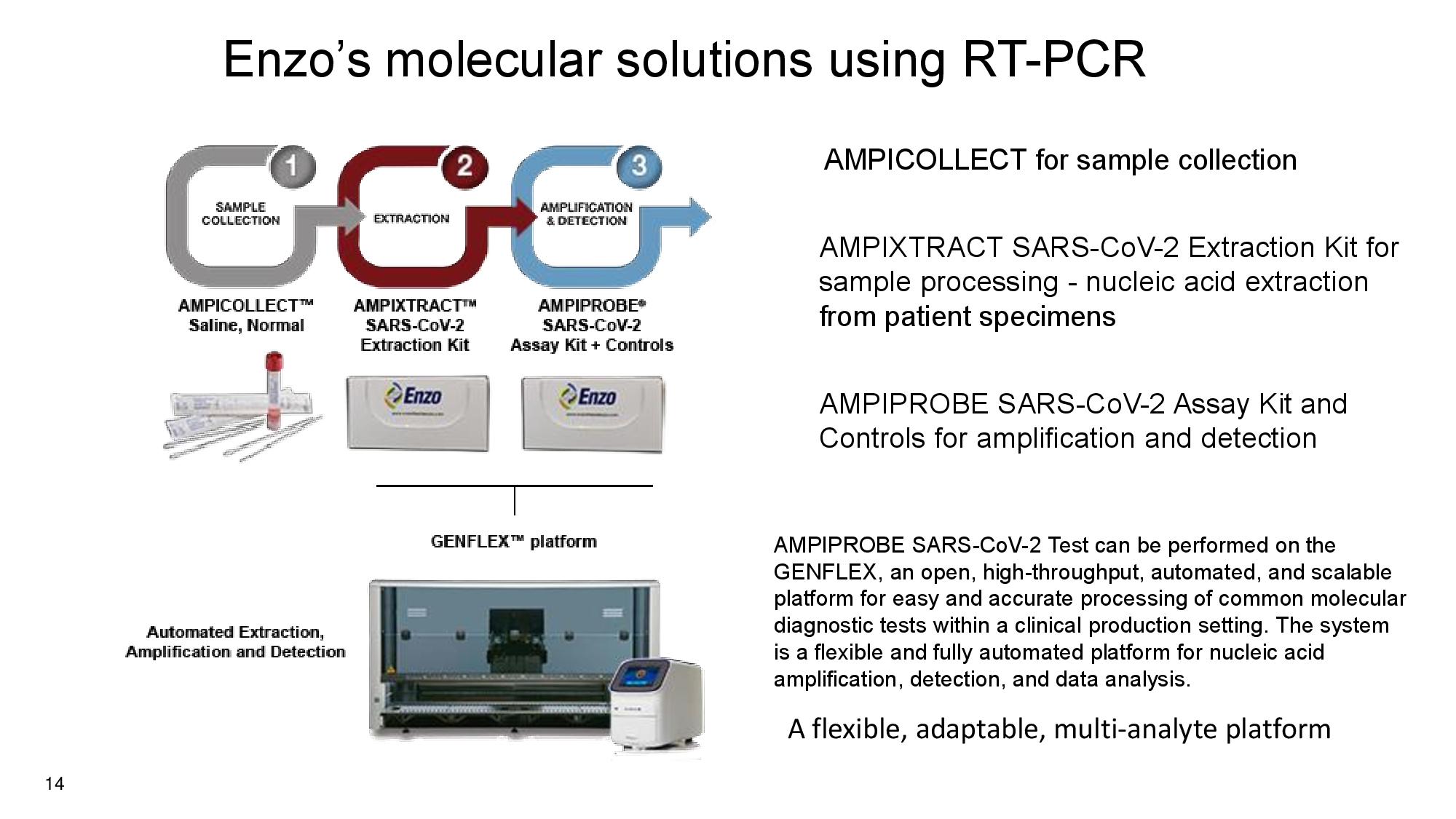
14 Enzo’s molecular solutions using RT - PCR AMPICOLLECT for sample collection AMPIXTRACT SARS - CoV - 2 Extraction Kit for sample processing - nucleic acid extraction from patient specimens AMPIPROBE SARS - CoV - 2 Assay Kit and Controls for amplification and detection AMPIPROBE SARS - CoV - 2 Test can be performed on the GENFLEX, an open, high - throughput, automated, and scalable platform for easy and accurate processing of common molecular diagnostic tests within a clinical production setting. The system is a flexible and fully automated platform for nucleic acid amplification, detection, and data analysis. A flexible, adaptable, multi - analyte platform
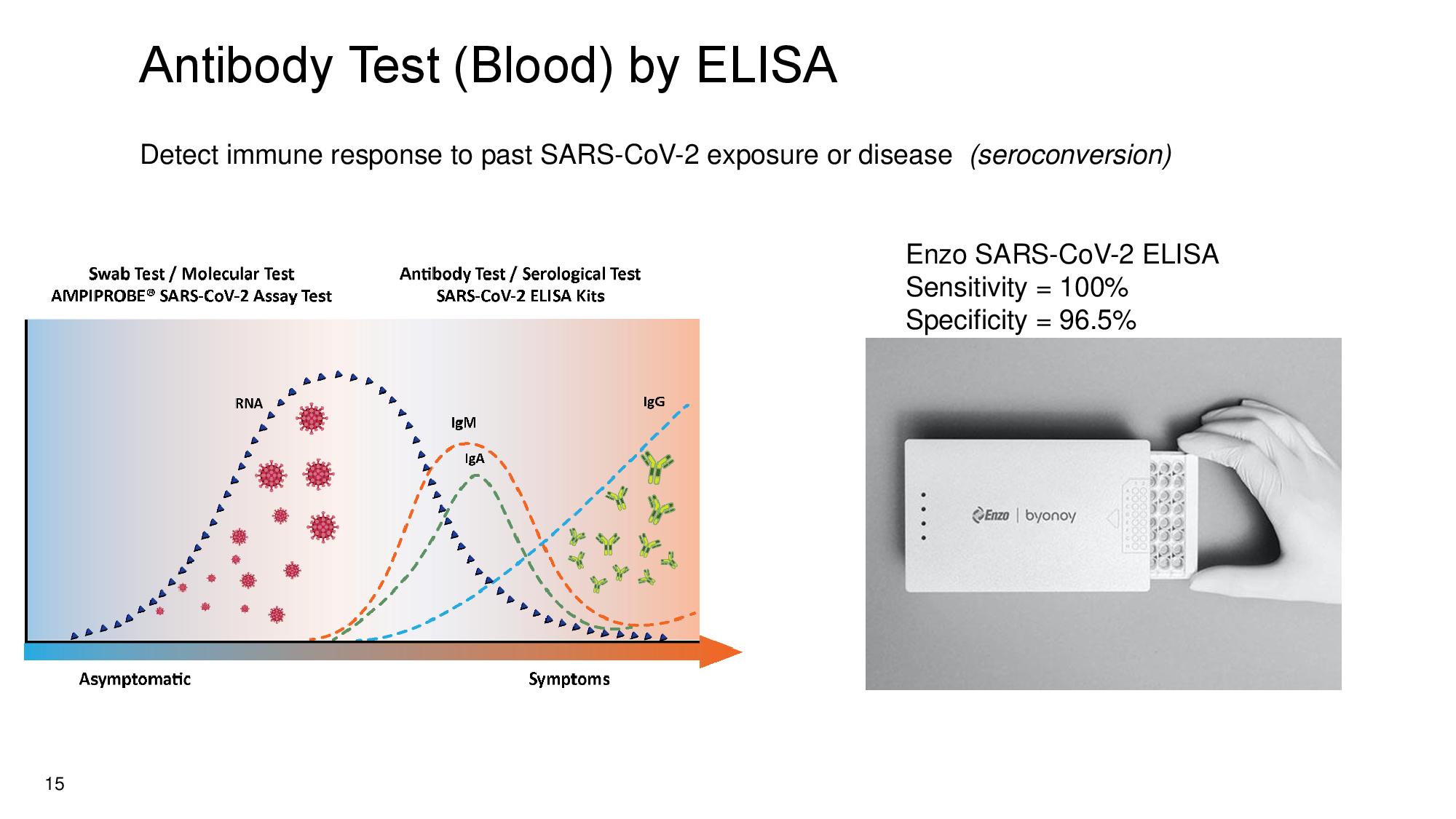
15 Antibody Test (Blood) by ELISA Detect immune response to past SARS - CoV - 2 exposure or disease (seroconversion) Enzo SARS - CoV - 2 ELISA Sensitivity = 100% Specificity = 96.5%

16 Issues Post Vaccine: How will testing algorithms be modified? • Vaccine acceptance : incidence of adverse reaction - time to herd immunity • Will the virus mutate : i.e. in humans or in the animal reservoir • Antibody testing will become standard of practice - do Ab positive patients, from prior exposure need vaccine? • Will testing be de - centralized : direct Antigen tests for detection of virus is limited by low sensitivity requires high numbers for reliable detection. = poor Negative Predicitive Value - (NPV) requiring NAAT confirmation • Cost to patient : - self - pay, third party payers, state/local governments • Who will be the ongoing test pool : worried well, travelers, contact tracing, returning workers/students, HCW’s, referred by HCP i.e. GoTestMeNow How will the vaccine impact the need for testing? What type of testing will be needed going forward?

17 17 Gerard Nuovo, MD Dr . Gerard Nuovo is a Professor of Pathology at the Wexner Medical Center at Ohio State University and a board - certified anatomic pathologist . He has spent his entire 30 + year career correlating the histologic features with the in situ detection of DNA, RNA, and proteins . He currently is medical director of a Molecular Pathology Laboratory (Phylogeny) that serves as a satellite laboratory for the OSUCCC . His group has invented several methodologies that can be used for the enhanced in situ detection of mRNA, viral nucleic acids, and microRNAs, including RT in situ PCR and the ultramer extension method for the latter . Further, he has extensive experience with the in - situ co - localization of RNA/DNA molecules and proteins . He has published over 333 peer review manuscripts, written 6 textbooks, has done over 40 invited chapters, and has been a co - PI on over 20 grants . His group was the first to show that human papillomavirus induced a type specific immunity and was one of the first groups to show that HIV - 1 caused a massive infection prior to the development of AIDS . He has done extensive work on the molecular events that underlie the use of viruses as oncolytic agents in cancer .
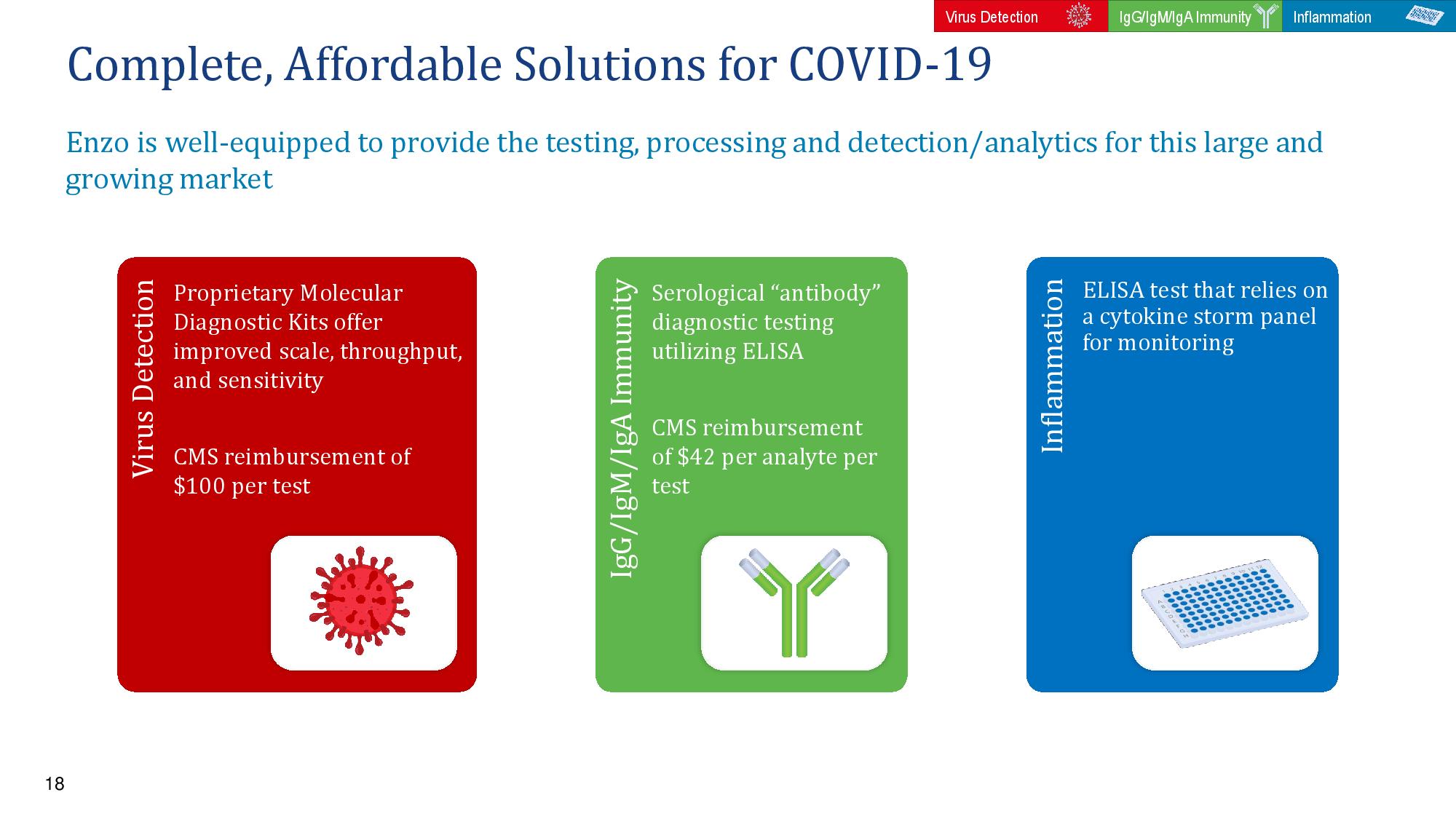
18 Complete, Affordable Solutions for COVID - 19 Enzo is well - equipped to provide the testing, processing and detection/analytics for this large and growing market Inflammation ELISA test that relies on a cytokine storm panel for monitoring Virus Detection Proprietary Molecular Diagnostic Kits offer improved scale, throughput, and sensitivity CMS reimbursement of $100 per test IgG/IgM/IgA Immunity Serological “antibody” diagnostic testing utilizing ELISA CMS reimbursement of $42 per analyte per test Virus Detection Inflammation IgG/IgM/IgA Immunity

19 Inflammation • Commercialization of full inflammation panel including detection of Interleukin 6 (IL - 6) levels to enable administration of immunosuppressant to treat Coronavirus patients demonstrating hyper immune response • Enzo 96 - well ELISA plate test is performed in a clinical lab using common lab workflow • Turnaround time is 24 hours and results are accurate and economical • Easily scalable Cytokine Storm Immunoassay for Inflammation Monitoring Cytokine Storm Immunoassay for Inflammation Monitoring Virus Detection IgG/IgM/IgA Immunity Inflammation 19

20 Pathophysiology of SARS - CoV2 infection Gerard Nuovo, MD and Cynthia Magro , MD (Professor of Pathology, Cornell Medical Center) • Initial infection in the nasopharynx by SARS - CoV2
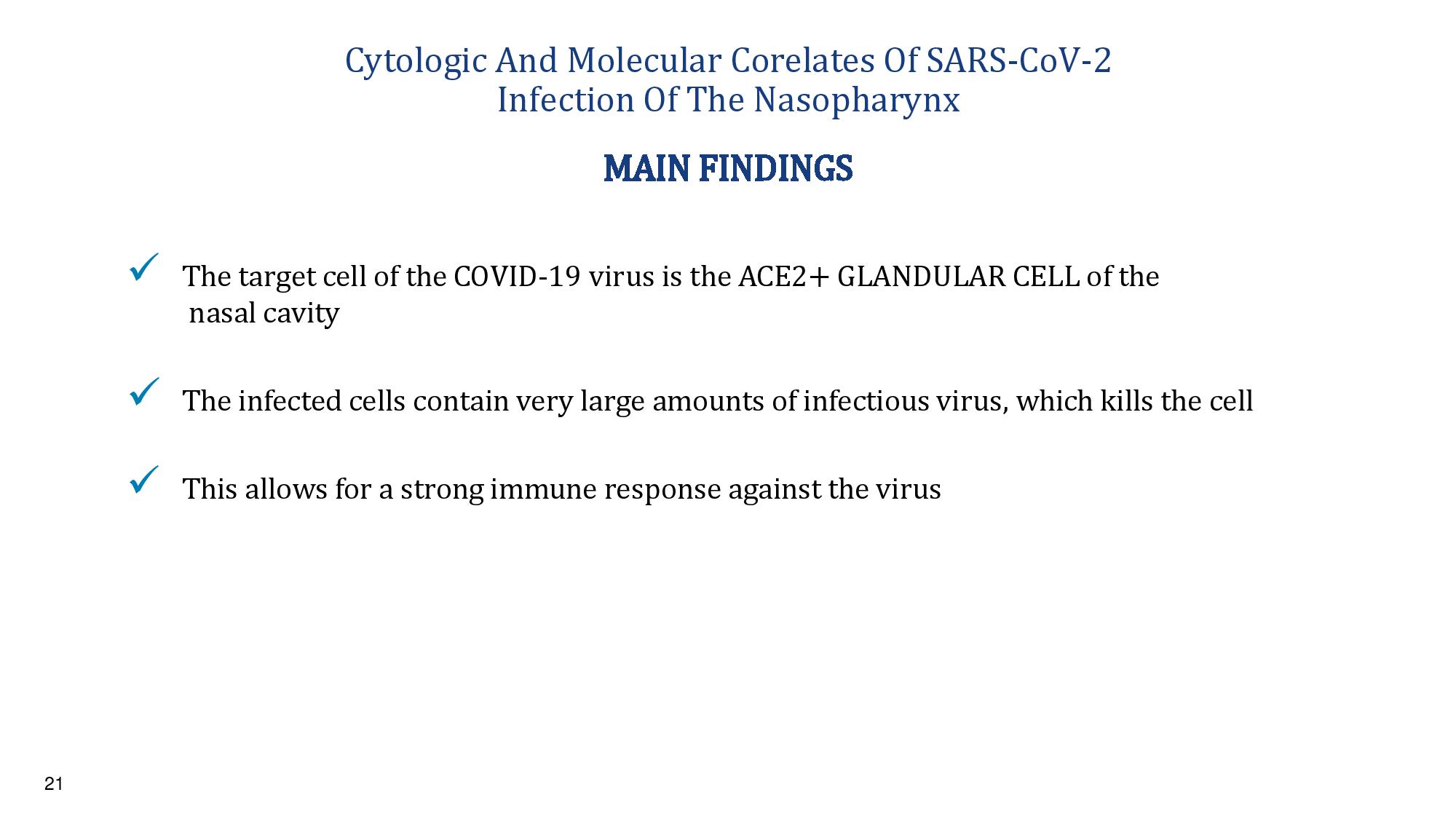
21 Cytologic And Molecular Corelates Of SARS - CoV - 2 Infection Of The Nasopharynx x The target cell of the COVID - 19 virus is the ACE2+ GLANDULAR CELL of the nasal cavity x The infected cells contain very large amounts of infectious virus, which kills the cell x This allows for a strong immune response against the virus MAIN FINDINGS
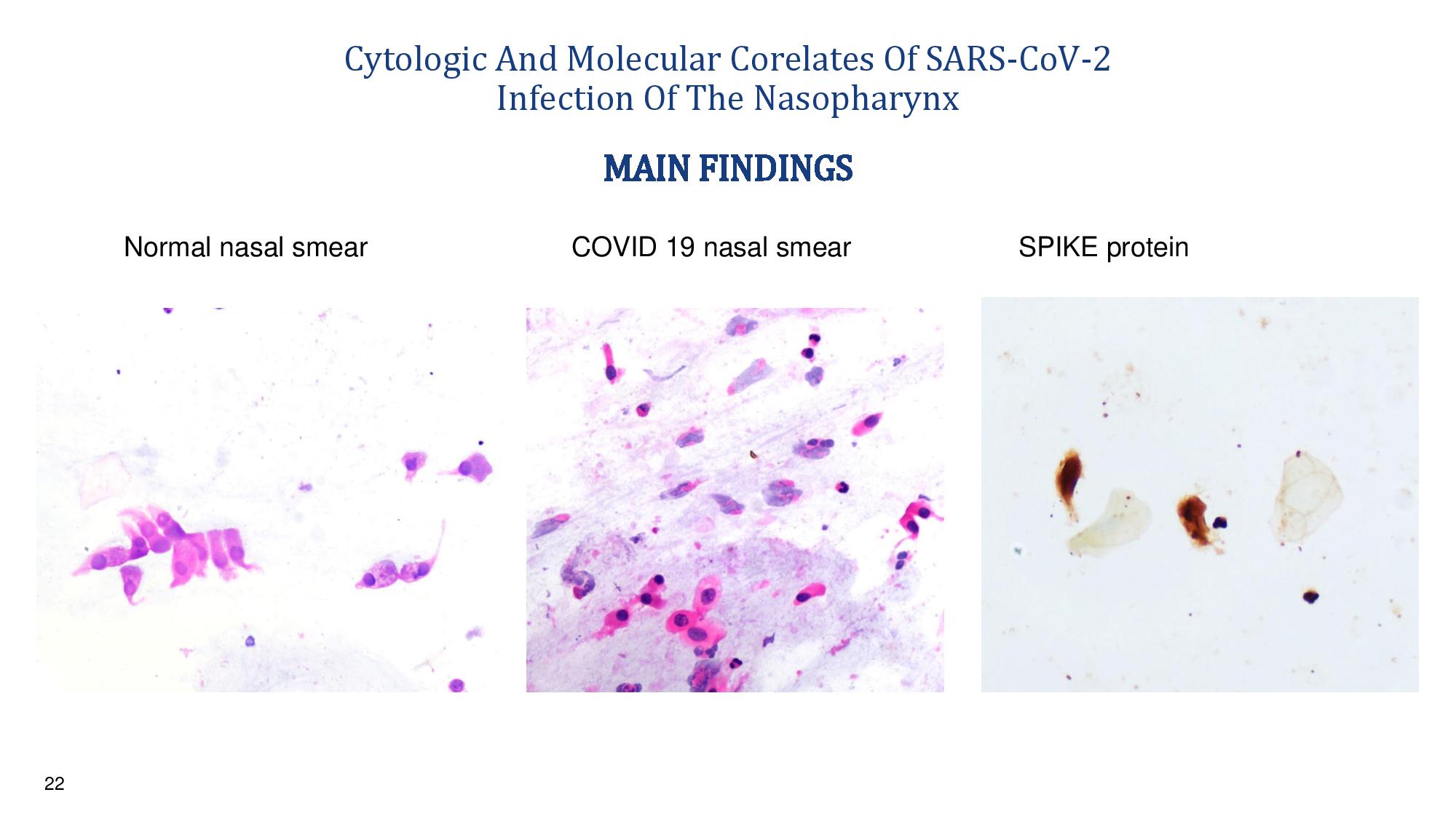
22 Cytologic And Molecular Corelates Of SARS - CoV - 2 Infection Of The Nasopharynx Normal nasal smear COVID 19 nasal smear SPIKE protein MAIN FINDINGS

23 Pathophysiology of SARS - CoV2 infection Gerard Nuovo, MD and Cynthia Magro , MD (Professor of Pathology, Cornell Medical Center) • Infection in lung associated with massive infection and complement mediated death of virus Translational Research 2020; 220:113)
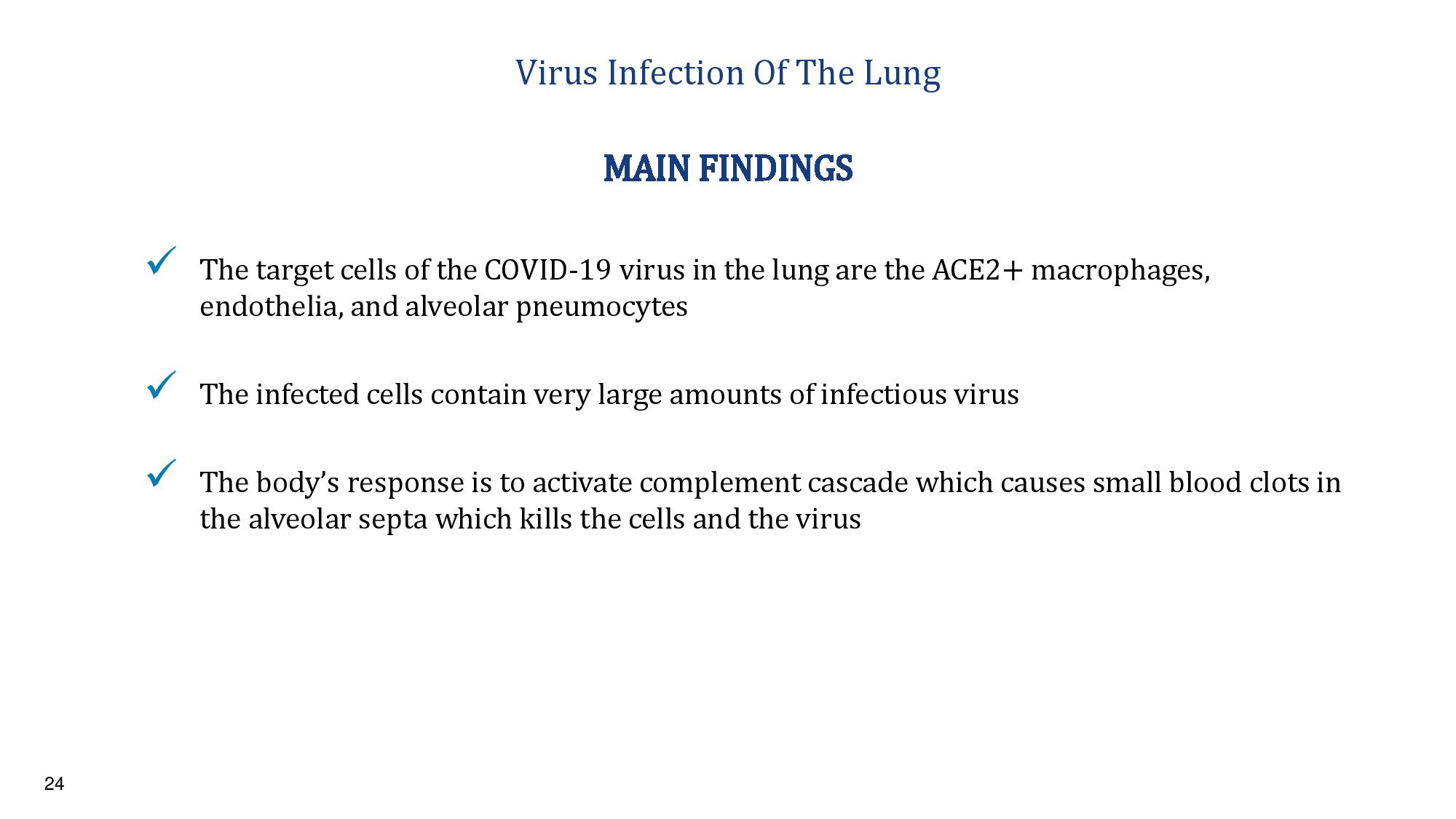
24 Virus Infection Of The Lung x The target cells of the COVID - 19 virus in the lung are the ACE2+ macrophages, endothelia, and alveolar pneumocytes x The infected cells contain very large amounts of infectious virus x The body’s response is to activate complement cascade which causes small blood clots in the alveolar septa which kills the cells and the virus MAIN FINDINGS
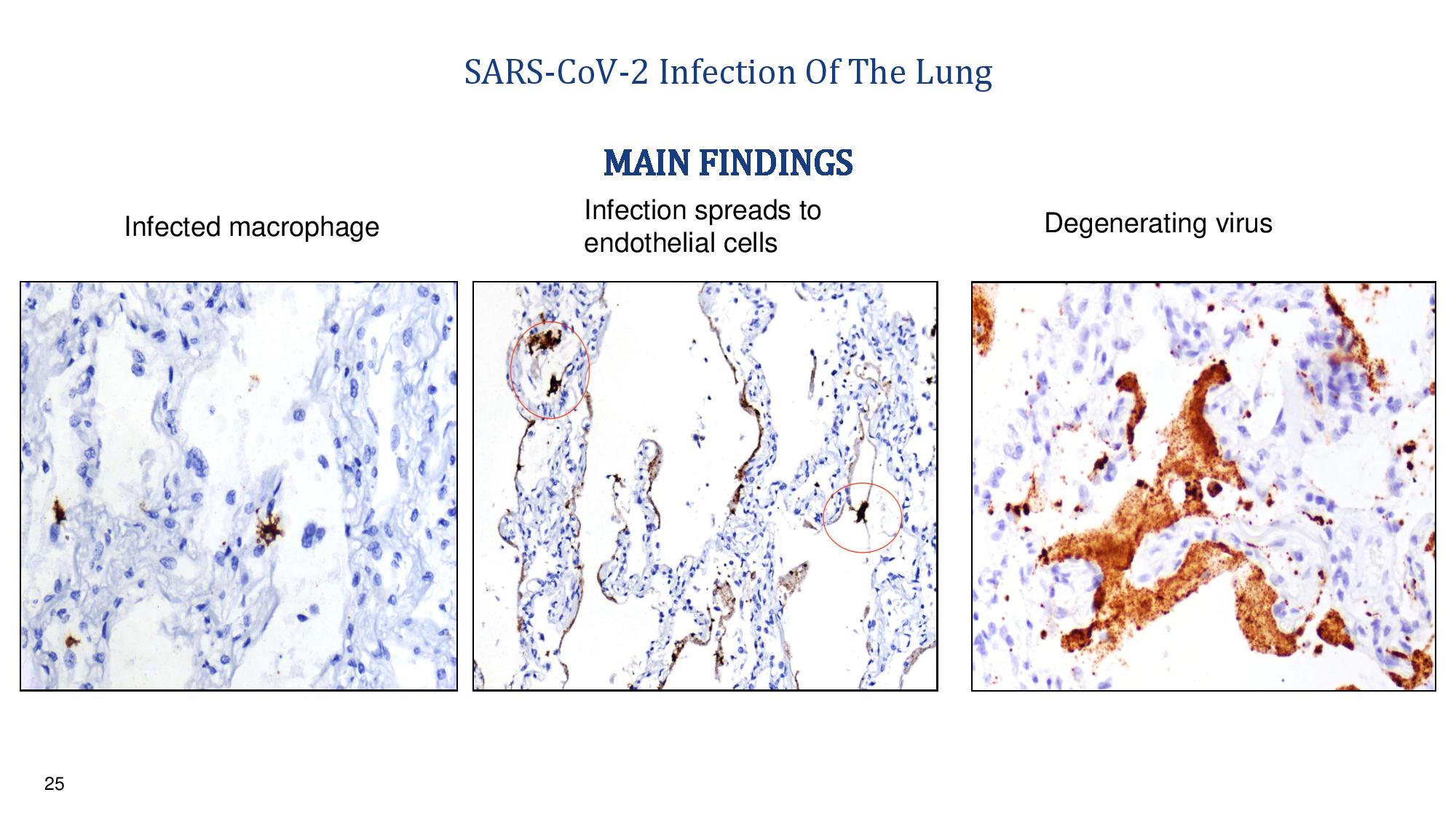
25 SARS - CoV - 2 Infection Of The Lung Infected macrophage Infection spreads to endothelial cells Degenerating virus MAIN FINDINGS
26 WHAT IS THE CONSEQUENCE OF THE DEGENERATED VIRAL PROTEINS ENTERING THE CIRCULATION?
27 Circulating Viral Proteins Can Cause A Lot Of Damage x Viral spike protein enters circulation and binds to ACE2+ endothelia in microvessels x The sites with the highest amount of ACE2+ endothelia are the SKIN/FAT and brain (also heart, liver, kidney) x The docked spike protein kills the endothelial cell, activates the complement cascade, and increases cytokine expression ( TNFa , IL6, IL8, IL1 beta). HENCE, THIS IS THE SOURCE OF THE INCREASED COAGULABILITY AND CYTOKINE STORM OF SEVERE COVID - 19 MAIN FINDINGS
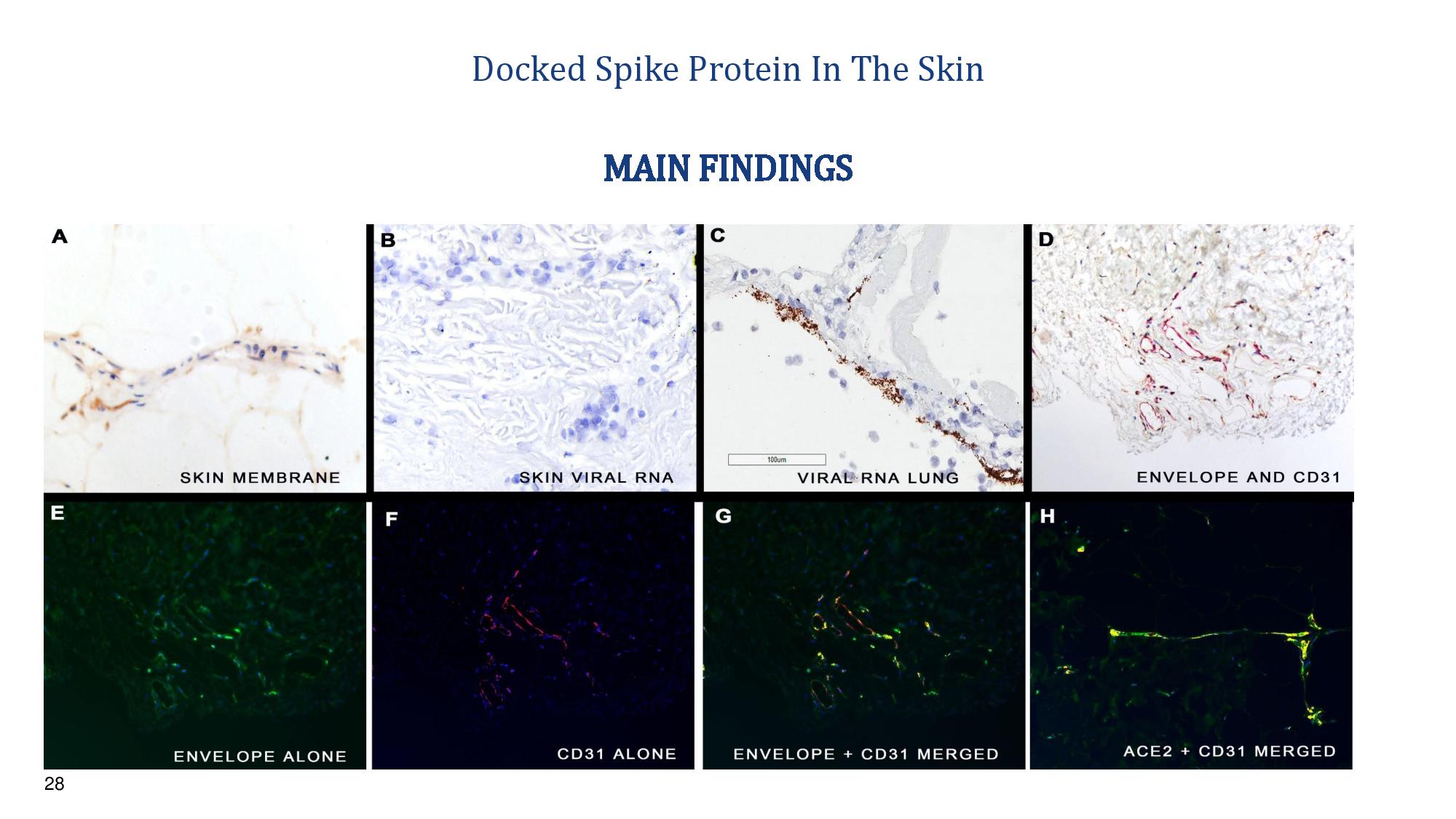
28 Docked Spike Protein In The Skin Viral capsid Viral RNA Viral RNA lung MAIN FINDINGS
29 WHAT IS THE CONSEQUENCE OF THE DEGENERATED VIRAL PROTEINS ENTERING THE CIRCULATION?
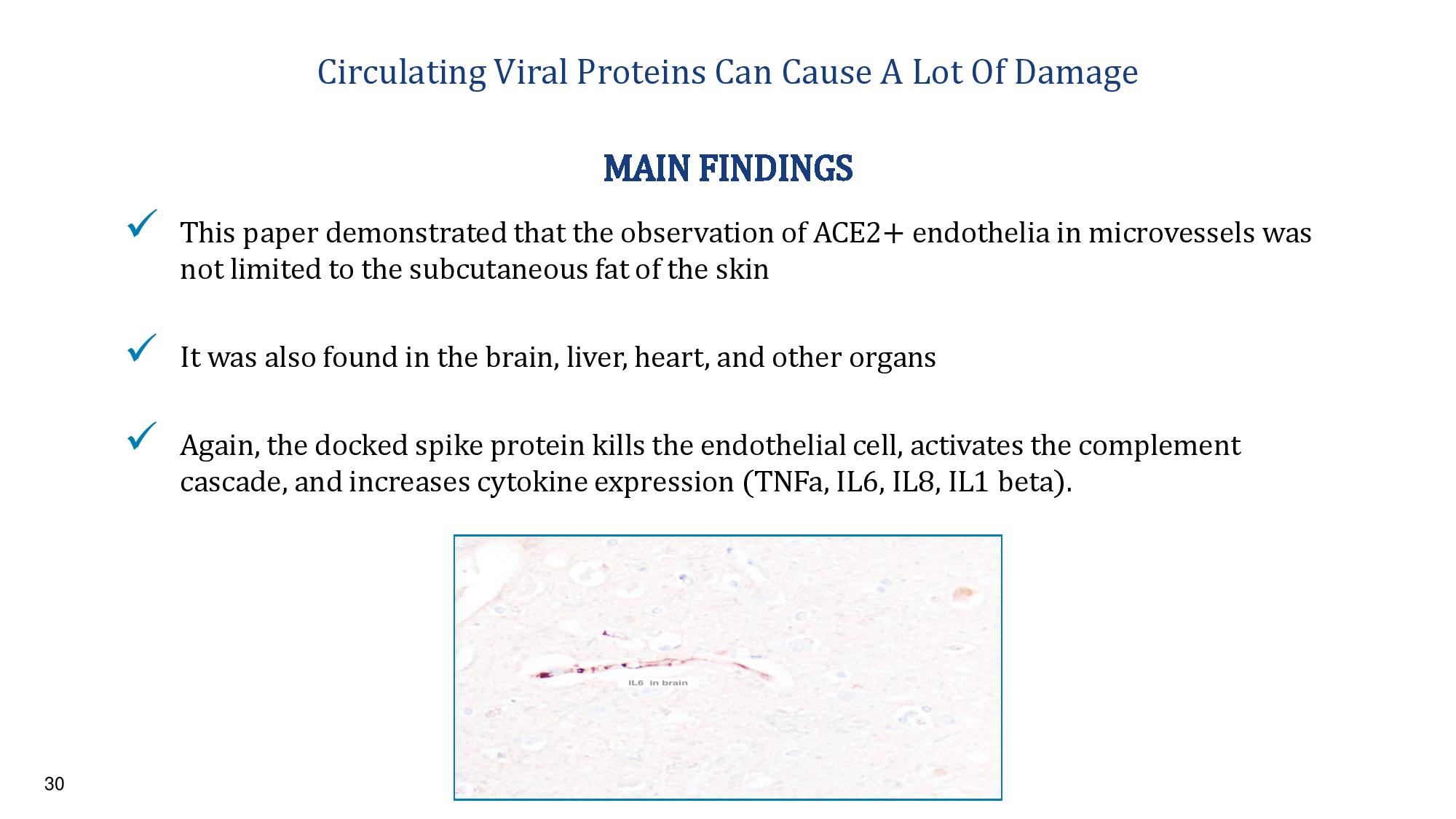
30 Circulating Viral Proteins Can Cause A Lot Of Damage x This paper demonstrated that the observation of ACE2+ endothelia in microvessels was not limited to the subcutaneous fat of the skin x It was also found in the brain, liver, heart, and other organs x Again, the docked spike protein kills the endothelial cell, activates the complement cascade, and increases cytokine expression ( TNFa , IL6, IL8, IL1 beta). MAIN FINDINGS
31 Conclusions x Large reservoirs of infectious virus are limited to the nasopharynx and lung x The microthrombi in lung kills virus, but this releases the spike (capsid) protein into the circulation (NOT infectious but still dangerous) x The docked spike protein kills the ACE2+ endothelial cell, activates the complement cascade, and increases cytokine expression (cytokine storm). If this could be detected at an early stage, and stopped, it would save many lives

Scientists Enabling Healthcare 32 Gerard Nuovo, MD Elazar Rabbani, PhD Bruce Hanna, PhD Questions & Answers

Scientists Enabling Healthcare Thank You www.enzo.com IR@enzo.com 33